Welcome to The American Cheese Society
ACS is the leader in promoting and supporting American cheeses, providing the cheese industry with educational resources and networking opportunities, while encouraging the highest standards of cheesemaking focused on safety and sustainability.
This site offers resources, education, industry directories, and other valuable tools for cheese professionals and enthusiasts alike. Membership in the American Cheese Society is available to anyone who enjoys great cheese: we hope you’ll explore our site and consider joining our community of more than 2,300 members in the United States and beyond!





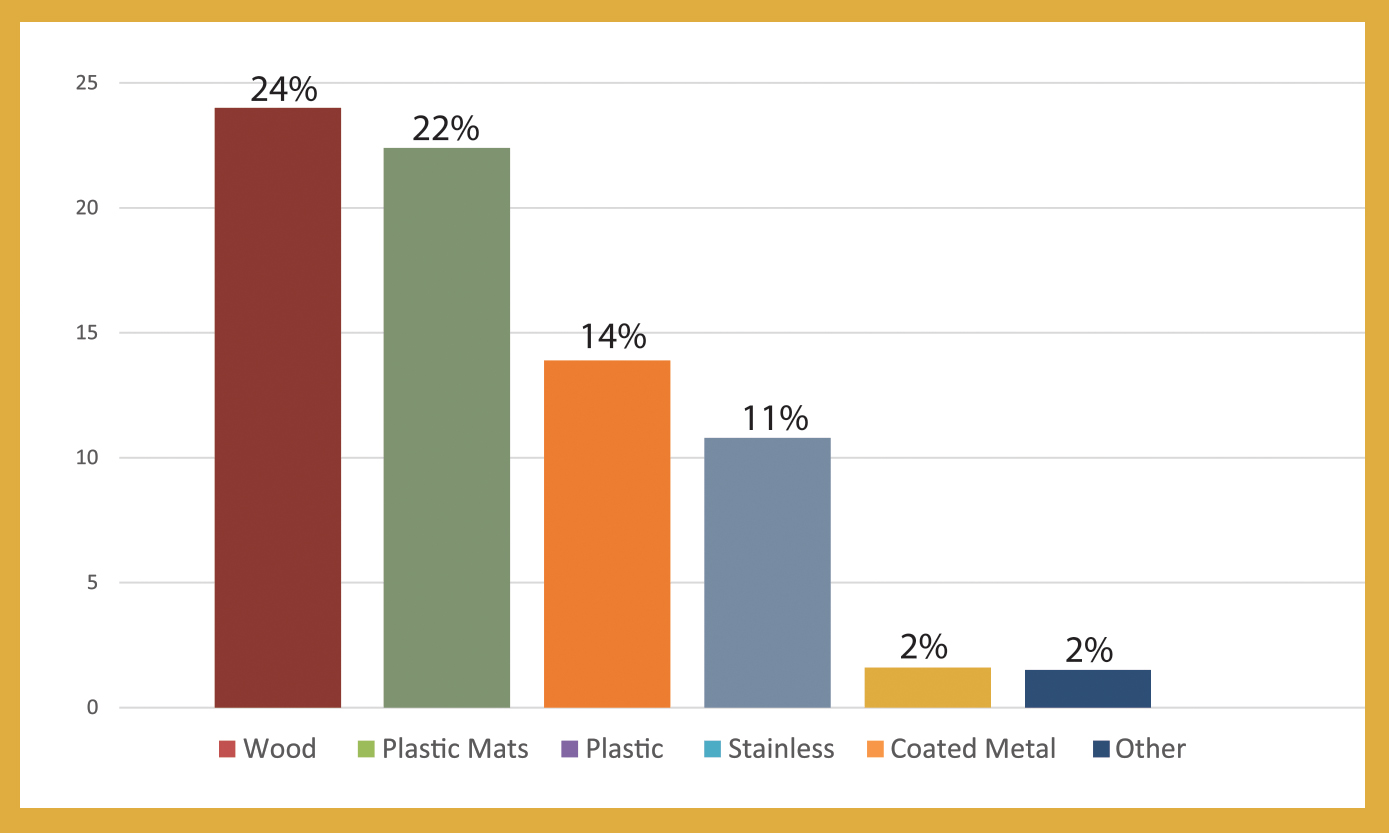 The 2020 State of the U.S. Artisan/Specialty Cheese Industry Survey Reports are now available. These reports expand significantly upon what has historically been a limited field of data available about US producers.
The 2020 State of the U.S. Artisan/Specialty Cheese Industry Survey Reports are now available. These reports expand significantly upon what has historically been a limited field of data available about US producers.

